Ionomers
Ion-Containing Polymers
General Properties
Ionomers are synthetic polyelectrolytes that consist of both electrically neutral and ionized groups that are randomly and or regularly distributed along the polymer backbone. They can be divided into polycations, polyanions and polyampholytes. Like ordinary polyelectrolytes, they can bear one or more charges depending on the pH value. However, per definition, their ion content is usually no more than 10 to 15% and, thus, most ionomers are insolube or only slightly soluble in water. One important characteristic of ionomers is the strong molecular aggregation of the ion-carrying groups to ion rich domains or ion clusters which act as physical crosslinks. When heated, the ionic bonds and clusters dissolve and when cooled, they reform. This gives ionomers a unique structure and behavior. At low temperatures they behave like crosslinked polymers (elastomers) and at elevated temperatures like ordinary thermoplastics.
The majority of ionomers studied have a polyvinyl or polydiene backbone and carry anionic groups (anionomers) with Na+, and Zn2+as counter ions. The three most important ionized groups are carboxylate (–COO-), sulfonate (–SO3-) and phosphonate (–PO32-) which differ in the strength of the ionic interaction. Usually, less than 80% of the acid groups are neutralized by a base. The remaining acid groups provide sites for hydrogen bonding between neighboring moelcules which are weaker than ionic bonds but stronger than secondary bonds. Thus, increasing the acid and ion content in the polymer results higher mechanical strength, modulus, and toughness. Carboxylate ionomers with an olefin backbone are by far the most common ionic polymers followed by sulfonate ionomers with a styrene backbone. Both types of ionomers are produced on a commercial scale and find many applications. Other backbones that have been studied include polybutadiene, polyacrylate, polymethacrylate, polyisoprene, and polytetrafluorethylene.
Ionomers can be either synthesized by copolymerization of ionic and neutral monomers or by chemical modification of electrically neutral polymers. The majority of ionomers are produced via free-radical copolymerization. For example, ethylene is copolymerized with methacrylic and/or acrylic acid by a high pressure process similar to low-density polyethylene. These ionic copolymers have typically a low melting point, improved toughness/flexibility and mechanical strength and when used as films, posess a much higher clarity and gloss and provide superior hot tack, seal strength and puncture resistance than unmodified polyethylene film. Another important class of carboxylate ionomers are copolymers of acrylates with acrylic and/or methacrylic acid, produced by free-radical emulsion or solution copolymerization. These ionomers are used as pressure-sensitive adhesives. A commercially important sulfonate ionomer is sulfonated tetrafluoroethylene (Nafion) which is produced by free-radical copolymerization of tetrafluoroethylene (TFE is the monomer of Teflon) with a perfluorinated vinyl ether sulfonyl fluoride co-monomer. The copolymers carries perfluoroether pendant side chains terminated by sulfonic acid groups. These ionomers have excellent chemical and thermal stability and can absorb large amounts of water. They are often used as ion-selective membranes. An ionomer that is produced via chemical modification is (lightly) sulfonated polystyrene. These ionomers are obtained by treating polystyrene with sulfonating agents such as acetyl sulfate in chlorinated solvents (post-sulfonation). This method can also be employed to produce block-copolymers with lightly sulfonated styrene blocks. The sulfonated ionomers form physical crosslinks that approach the strength of covalent links which is desirable in many elastomeric applications.
COMMERCIAL Ionomers
The largest volume ionomers are copolymers of ethylene and acrylic and/or methacrylic acid. Commercial grades of ethylene (meth)acrylic acid (EAA, EMAA) are available from DuPont (Surlyn®, Nucrel®), SK Global Chemical1 (Primacor™), and Ineos, (Eltex®).
Perfluorinated sulfonic acid ionomers (PFSA) are sold by DuPont (Nafion), Solfay (Aquivion), Chemours, and 3M.
| Ionomers | Structure of Repeat Unit |
| Poly(ethylene-co-methacrylic acid) Ionomer (Na, Zn) |
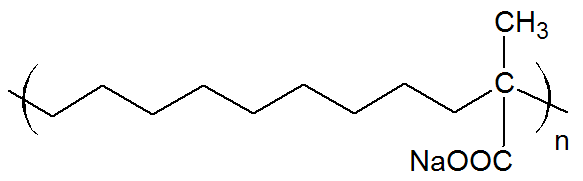
|
| Poly(ethylene-co-acrylic acid) Ionomer (Na, Zn) |
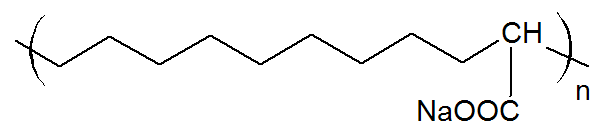
|
| Perfluorinated sulfonic acid ionomers (PFSA), Nafion |
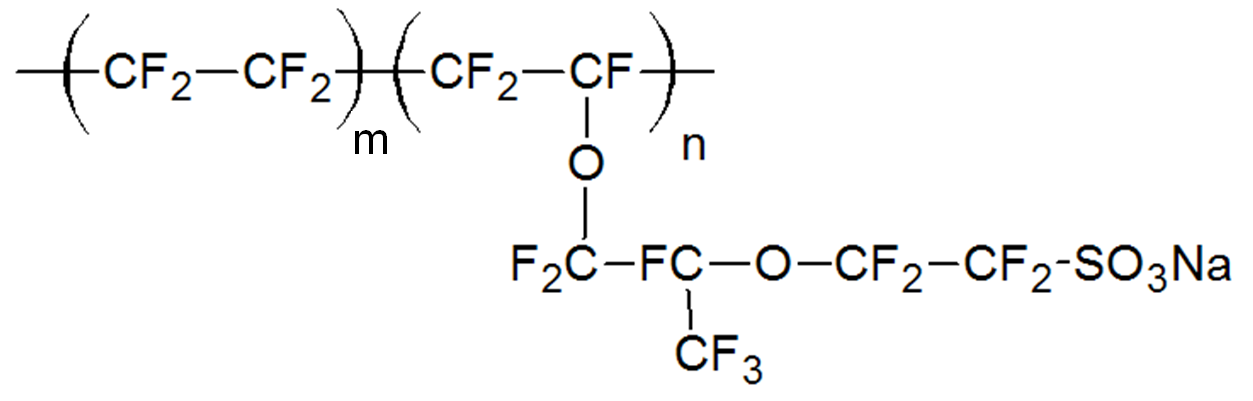
|
Applications
The by far most important ionomer is ethylene acrylic acid copolymer (EAA) which is sold under the tradename Surlyn by DuPont. It is frequently used as a food packaging material and as a tie-layer (compatibilizer) in multi-layer films. Other important applications include coatings and surface films for golf balls, sports equipment, and for overmolded (cosmetic) bottles.
Perfluorinated sulfonic acid ionomers (Nafion) are often used as ion-selective membranes. Important applications include cation exchange membrane for fuel cells, PEM water electroyzers, separators for redox flow batteries, electrodialysis, and electrochemical hydrogen compressors.
1SK Innovation, the energy solution unit of the South Korean conglomerate SK Group, took over Dow Chemical's EAA business at $ 370MM. Dow divested its copolymers unit to gain antitrust approval for its merger with the US chemical competitor DuPont. (PULSE, February 2, 2017)